Science, Reason and a chance for Compassion
by Owen Smith
Designated Growers in BC can legally make Extracts
On May 31st 2013 Judge Johnston extended his decision from my Voir Dire (trial within a trial) in 2012, granting Designated Growers (DG’s) in British Columbia the right to make cannabis derivative products like hash, oils and butters for their authorized patient(s). This decision came on an auspicious day for me: May 31st is my sister’s birthday. My sister Ceri died seven years ago of melanoma skin cancer. At the time, I didn’t know of the potential of cannabis as medicine beyond smoking it to help her through chemotherapy treatment stimulate her appetite, sleep and laugh with her family and friends, truly miraculous effects regardless of the outcome. I have learnt a great deal since she passed and believe now that she may have seen benefit from eating and/or using cannabis extracts on her skin.
As with my sister, the majority of medical cannabis users come to cannabis only after exhausting the treatment options offered to them by their physician. These people often need assistance as they are experiencing debilitating side effects from their medications. It is an unfair to the conscience of this person that he or she is by necessary compassion, compelling a person who cares for them to break the law in order to make them suitable medical products. This latent enforcement potential is unnecessarily focused on those with seriously disabling conditions and particularly threatens those compassionate people who aid them.
I’m deeply pleased that at least people in BC, who are thoroughly exhausted by their severe medical conditions, can have a crystal clear conscience as their family member or friend makes and/or helps them apply their medicine. I hope this provokes a wave of compassionate action, inspiring DG’s (and maybe soon LP’s) to advance their knowledge of cannabis medicines by helping them to legally provide smarter, higher quality, medicinal products.
Government comtempt
At the conclusion of the Voir Dire, my awesome lawyer Kirk Tousaw, who has fought other medical cannabis constitutional challenges, argued that the government has shown contempt for the courts by not adequately responding to previous court orders and predicted the same fate for Judge Johnston’s ruling. Johnston said in the final words of his decision, “a court should be slow to attribute such bad faith or motive to legislative response to court decisions.”[i] However, by taking no action in the past year to respond to Johnston’s ruling, the government is confirming our argument and edging themselves closer to contempt.
At the May 31st hearing, Johnston said that there was little pretence the Crown’s application was an attempt to preserve the status quo until the BC Court of Appeal hears the case on Oct. 17. He said he would proceed on the basis that he made the right decision[ii]. This window of opportunity for DG’s in BC will remain open until the ruling of the BC Court of Appeal sometime in early 2014. By ignoring the Courts’ order, Health Canada has put the constitutionality of their new program in immediate question. The BC Court of Appeal may deliver their ruling any time around the implementation date of the MMPR, April 1st 2014.
Health Canada’s recent release of a revised medical cannabis program, the MMPR contains no reference to Judge Johnston’s decision and only affords a brief consideration for extracts amid the responses to stakeholder concerns during the brief consultation period. “The new Regulations will limit licensed producers to the production and distribution of dried marihuana only. The MMPR will not authorize extractions of active ingredients (e.g. resin) to be sold for the therapeutic purposes”[iii]. They also say, somewhat confusingly, that “There are no restrictions on how dried marihuana is to be ingested or inhaled, and patients may choose to use it, for example in foods or by vaporizing. HC does not limit or recommend a particular method of administration.”[iv]
Patient Criminals
The government had argued in the Voir Dire that to extract the resin from the plant material and store it any way would handcuff the police officers who would ordinarily quantify dried cannabis to judge if a patient is within their legal limit. They argued that the presence of an unquantifiable extract would increase the risk of diversion to the black market.
In his ruling Judge Johnston wrote that if Health Canada already permits patients to “bake their dried plant material into a cookie batter or any other food, mix it into a salve, or otherwise deal with it in a similar fashion, so long as they used it as dried material” that “there is little ‘rational connection’ between the restriction to dried marihuana and the legitimate objective of preventing diversion of lawful medical marihuana into the illegal market.”[v]
“If it is possible to distinguish chopped up, dried marihuana from other dried plant material such as might be found in most kitchen spice jars, it seems to me that there should have been evidence led on the point. I am not prepared to infer that it is necessary to restrict medical marihuana to its dried form in order to make enforcement of the drug laws possible. I am not concerned with making enforcement of the drug laws easy if the cost of doing so puts the rights protected by s. 7 of the Charter at risk. In the absence of clear evidence that the restriction to dried marihuana is necessary, I conclude that this restriction is arbitrary.”[vi]
“The restriction to dried marihuana unnecessarily, and therefore to an unreasonable degree, impairs the security right to choose how to ingest the medicinal ingredients in the safest and most effective manner”[vii]. Johnston’s decision was largely informed by Dr. David Pate. Pate was able to clarify the botanical facts that make extracting cannabis a safe, effective and logical step in a medical preparation (the complete transcripts of the trial are available online here[viii]).
Resin science
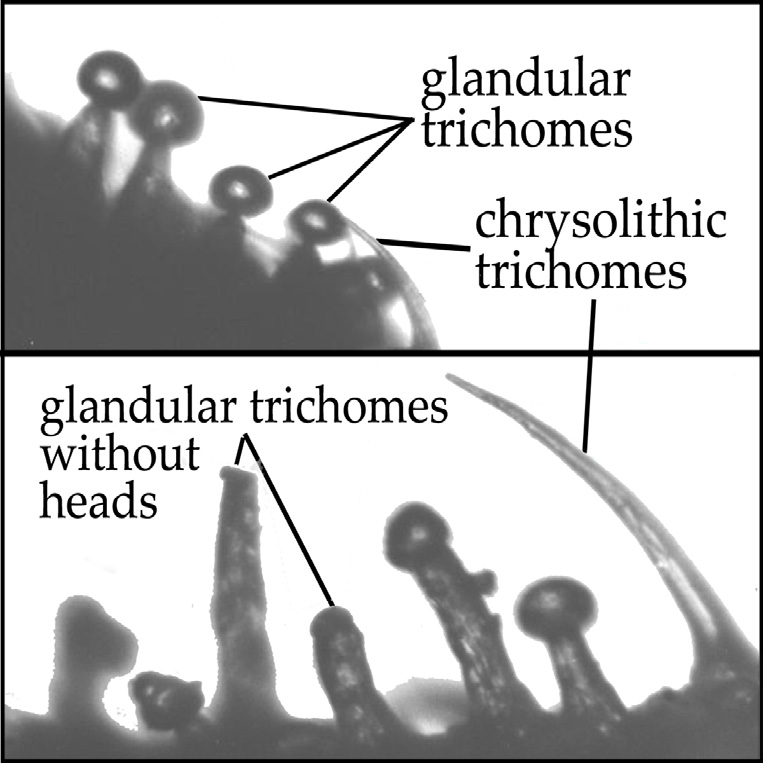
Other than the well known glandular trichomes (that I diagram in the article Concentrating on Cannabinoids), there are cystolithic trichomes distributed all over the plant surface. “Cystolithic trichomes [are] non-glandular trichomes which contain a little crystal [of] oxalic acid at their base which is perhaps unpalatable. It’s speculative as to why it exists there, but the general thought is that all of these things are defensive strategies against insect predation or even mammals.”[ix]
As plants can’t run away, they develop mechanisms to subvert predation in the competitive wilds of nature; some of the cannabis plants’ compounds and mechanisms function to fulfil these roles, making them undesirable in a medical preparation. Due to the structural difference between the two kinds of trichome, simple extraction techniques can separate the medicine held in the gland from the deterrent stored in the chrysolith. Glandular trichomes are formed with a long thin neck pretentiously supporting a globular head full of Cannabinoid rich resin while chrysolithic trichomes are a sturdier thorn shape (see figure 1).
[figure 1. Comparison of crysolithic and glandular trichomes]
The resin glands separate so easily from the dried plant that in the act of sending a package in the mail, the singular dispensing method proposed under the MMPR, an amount of resin will be separated and collected at the bottom of the package. Ultimately, there is no reason to disallow the resin glands that are attached to a plant, after previously allowing the plant that is covered in these glands for the purpose of obtaining the desired compounds from within them.
The MMPR document states that “there are no clinical studies on the use of cannabis edible (e.g. cookies, baked goods) or topical products for therapeutic purposes.”[x]
Dr. Pate was the head of a laboratory that developed the plants that are used to make Sativex, a whole plant Cannabis extract that has undergone clinical trials and is available by prescription in Canada. In addition Dr. Pate suggests that “cannabis has undergone a multiyear open label clinical trial by virtue of being in such popular use both medically and recreationally for an extended period of time, essentially hundreds of years.”[xi]
“The old style extracts were usually ethanolic extracts in a dropping bottle of some sort. You’d put a little in your tea or under your tongue. And the new version is the same extract in a spray bottle that you spray in your mouth. You could say that the new version, Sativex, is the historical vindication of the old materials that disappeared probably before their time.[xii]”
A Resin Resurgence
British Columbia has more DG’s than all the other provinces combined[xiii]. These skilful folks can easily move beyond the fine art of producing high quality dried cannabis buds to create tinctures like Sativex and more.
Derivatives can be produced from a previously wasted resource (leaf and stalk) by heating and soaking the material in oils which can be used to make a diversity of edible and topical products. Decarboxylation carefully activates the dormant Cannabinoids, increasing the effectiveness of the recovered material. The V-CBC recipe book has a detailed description of this simple process[xiv]
This expansion in the legal capacity of compassionate gardeners may improve access and help reduce the price by maximizing the utility of the plant. Creative DG’s will quickly grasp the many facets of the art and produce more medicines that are better suited for individual needs at little additional cost.
Smoking cannabis flower buds is the least efficient and most expensive way to consume Cannabinoids. The intense heat involved in smoking consumes up to 70% of the desired ingredients (see the article Product Testing) and creates undesired products from the combustion of the plant cellulose. Dried plant matter is integral to the act of smoking as, “most of the fuel component of the pyrolysis comes from burning the cellulose. That produces heat from the ember which superheats the air drawn through the ember and essentially both decarboxylates and distils the Cannabinoids out the downstream end.”[xv] More and more people are turning to vaporizers for a cleaner and more economical way to achieve the instant relief that inhalation can provide. The brown material that is left over can still be infused into vegetable oil.
The effects of eating cannabis persist for hours longer than inhalation. Edible products target the gastro-intestinal system and can be designed for either day time or night time use at a variety of strengths. Topical products can be applied directly to the area of need, which is especially appropriate for skin conditions like lesions. Establishing multiple application methods can allow patients to achieve a certain therapeutic effect which allows them to gain greater efficacy from smaller quantities of inhaled cannabis.
Dr. Pate expanded that when eating cannabis “you’re establishing a baseline level and if that’s sufficient, so be it. But if not, a much smaller amount through smoking can push those blood levels in a transient acute sense ‑- a quick onset and ‑- short diminution back to the levels ‑- back to the plateau provided by the oral ingestion. In other words it’s a peak on top of an already established plateau, but it’s more transient in nature.”[xvi]
MMAR patients, who face the removal of their right to grow their own plants or have someone grow for them, are facing the terrifying projection that their medicine costs will skyrocket far beyond their means. While there is a concerted effort to prevent this fundamental right from disappearing[xvii], if the new licensed commercial production facilities under the MMPR also gain the ability to produce derivatives, there may be avenues available to decrease the medicine costs and increase overall efficacy of this program for many seriously ill people.
Beyond this ongoing melee of courts, cops, cannabis clubs and Health Canada, caregivers and critically ill Canadians in BC may now be sharing these natural medicines without fear of persecution, carefully combating the conditions of their friends or family members. With this collective creative ingenuity, products may be tailored to specific diets: organic, vegan, gluten-free, sugar-free, dairy-free, all of the above and more; for specific effects: stimulating or relaxing and in a variety of dose strengths.
While carving out this medicine craft for Canadians, the products of the well established cannabis industries south of the border offer many good examples to follow. The internet hosts a massive bank of helpful medical cannabis videos and articles through which I am endlessly browsing. I encourage medicine makers to engage these communities to share what they learn so that together we may better serve the people who need our help. Some of these products may prove very useful to many patients in the future. Conservative estimates state that the number of people in the national medical cannabis program will grow ten fold to 430,000 in the next decade [xviii].
Visit Cannabisdigest.ca to read past articles and leave a comment.
[i] Regina v. Owen Edward Smith, Reasons for Judgment On Voir Dire. 133
http://canlii.ca/en/bc/bcsc/doc/2012/2012bcsc544/2012bcsc544.html
[ii] Judge dismisses bid for extension on medical marijuana changes
[iii] Marihuana for Medical Purposes Regulations. Consultations.
http://www.gazette.gc.ca/rp-pr/p2/2013/2013-06-19/html/sor-dors119-eng.php.
[iv] Marihuana for Medical Purposes Regulations. Consultations.
[v] Regina v. Owen Edward Smith, Reasons for Judgment On Voir Dire. 121, 122
[vi] Regina v. Owen Edward Smith, Reasons for Judgment On Voir Dire. 120
[vii] Regina v. Owen Edward Smith, Reasons for Judgment On Voir Dire. 123
[viii] R.v.Smith Transcripts. 2012.
http://www.scribd.com/collections/4243500/R-v-Smith-BC-Supreme-Court-2012-Transcripts
[ix] R.v.Smith Transcripts. 2012. V.2, p. 309, line 11
[x] Marihuana for Medical Purposes Regulations. Consultations.
[xi] R.v.Smith Transcripts 2012. V.2, p. 246 Line 17
[xii] R.v.Smith Transcripts. 2012. V.2, p. 351, line 26
[xiii] Marihuana Medical Access Program Statistics. Jan, 2012
http://www.hc-sc.gc.ca/dhp-mps/marihuana/stat/index-eng.php#a1
[xv] R.v.Smith Transcripts. 2012. V.2, p. 328, Line 15
[xvi] R.v.Smith Transcripts. 2012. V.2, p. 335, line 1
[xviii] M.M.P.R. Dec, 15 2012. Projected number of potential users
http://gazette.gc.ca/rp-pr/p1/2012/2012-12-15/html/reg4-eng.html